Chemistry Matter Mass Number. Look on the periodic table.
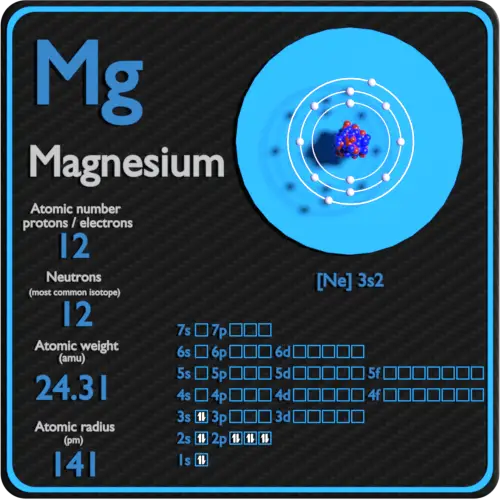
Magnesium Protons Neutrons Electrons Electron Configuration
What is its atomic number.

. An ion has 26 protons 28 neutrons and 24 electrons. An ion has 26 protons 30 neutrons and 24 electrons. Charge of ion 26 protons - 24 electrons 2.
It is element number 16 since that is the number of protons and electrons. Ion has 24 electrons so it has experienced loss of 2 electrons. Xe Ni Fe Mg Cr.
Atomic number Number of electrons. Since an atom is electrically neutral so the atom has 26 electrons also. A certain atom has 26 protons 26 electrons and 30 neutrons.
Write the symbol of ions. 26 protons and 24 electrons. A model of an atom shows eight electrons in rings that represent different energy levels.
The symbol for the ion is. Number of proton 26. The atomic mass is in the upper right corner.
An atomion has 26 protons 28 neutrons and 24 electrons. 0-2 24 52 28 54 O 2 26. How many electrons are in each energy.
C You can find the atomic mass by adding the number of protons and. The number of electrons in an ion with 20 protons and an ionic charge of -2 is. What is its mass number.
Number of electrons in Fe 2 26-2 24. 1 Answer anor277 Jan 28 2017 Its mass number is 56. Chemistry questions and answers.
I know sulphur has 16 of each. An ion has 26 protons 28 neutrons and 24 electrons. Chemistry questions and answers.
Also the element with 26 protons 26 atomic number is iron Fe. This means that the charge of the ion can be calculated as follows. Mass number is simply the total number of massive particles in the atom protons and neutrons.
The atomic number is by definiton the number of protons in an. So charge on ion is 2. A26 electrons29 neutrons26 protons b53 protons74neutrons c2electronsneutral atom d20 protons e86electrons125neutrons82protonscharged atom f0 neutrons.
For example if an ion has 9 electrons and 8 protons then it has a charge of -1. With 26 neutrons the mass number of this isotope is 50. Electrons have a negative charge.
Which element is this ion. Which element has 16 protons 17 neutrons and 16 electrons. If the substance has 24 protons per atom then it must be an atom of chromium but since the number of electrons is less than the number of protons the atom has an ionic charge of 2.
Hence option B is correct. According to this question an ion has 26 protons and 24 electrons. A neutral atom with atomic number 20 will have 20 electrons.
Question 4 1 pts An ion has 26 protons 29 neutrons and 23 electrons. Therefore if an ion has 26 protons and 24 electrons the charge of the ion will be 2. The symbol for the ion is.
Also asked what is the atomic number of an atom that has 26 protons 24 neutrons and 25 electrons. An ion from a given element has 26 protons and 24 electrons. 52Cu3 5573- 55Fe3 52Cu3- 55Fe3- D Question 5 1 pts An atom that has an atomic number of 38 and a mass number of 88 is an isotope of an atom that has an atomic number.
Answer 1 of 6. See the answer See the answer done loading. There are 26.
Fe2 The element potassium forms a with the charge The symbol for this ion is and the name is The number of electrons in this ion is isited Submit Answer Retry Entire Question. 112 rows Titanium has 22 protons 26 neutrons and 22 electrons. Protons and electrons both have a mass of 1 AMU.
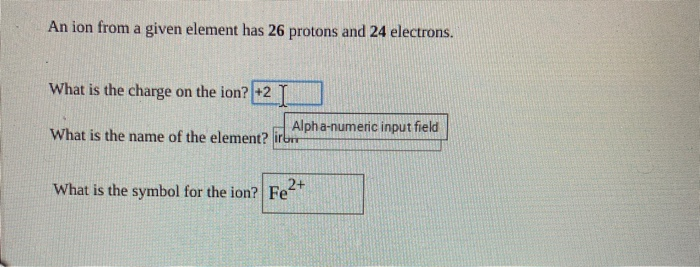
Solved An Ion From A Given Element Has 26 Protons And 24 Chegg Com
What Is The Symbol For The Species That Contains 24 Protons 26 Neutrons And 22 Electrons Quora

Unit Ii Atoms And The Periodic Table Ppt Video Online Download
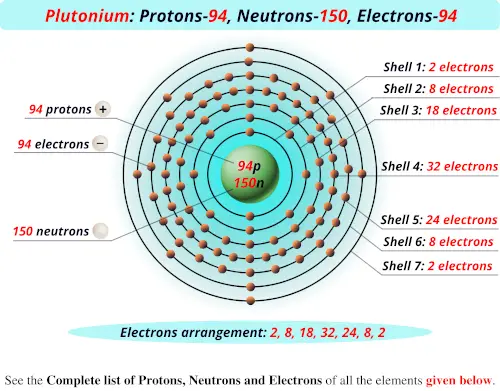
0 Comments